The Global Point-of-Care Molecular Diagnostics Market is poised for significant growth, expected to reach USD 11.3 billion by 2033 from USD 8.3 billion in 2023, reflecting a CAGR of 3.1% during the forecast period from 2024 to 2033. This growth is driven by several factors, including the increasing prevalence of infectious diseases and chronic conditions, which necessitate rapid and accurate diagnostic solutions. Technological advancements, particularly in the miniaturization and portability of diagnostic devices, have enhanced accessibility and efficiency, enabling healthcare providers to perform molecular-level tests even in remote or resource-limited settings.
However, the market faces challenges such as quality control issues and the high costs associated with point-of-care molecular diagnostics, which can hinder adoption in resource-constrained environments. Despite these hurdles, recent developments in the market are promising. For instance, the integration of mobile health (mHealth) solutions has improved the connectivity and real-time data transmission of diagnostic results, facilitating remote consultations and prompt clinical decisions.
The market is also witnessing significant advancements in PCR-based and genetic sequencing technologies, which are essential for detecting infectious agents and genetic mutations rapidly. These technologies are crucial in managing and containing outbreaks, as demonstrated by the COVID-19 pandemic, which highlighted the urgent need for swift and accurate diagnostic capabilities. Major players such as Abbott Laboratories, Roche Diagnostics, and BioMerieux are actively developing innovative products and expanding their market presence through strategic initiatives like mergers and acquisitions.
Overall, the increasing demand for quick diagnostic solutions, coupled with continuous technological innovations, positions the point-of-care molecular diagnostics market for robust growth in the coming years.
Key Takeaways
- The market is anticipated to grow from USD 8.3 billion in 2023 to USD 11.3 billion by 2033, with a CAGR of 3.1%.
- PCR-based diagnostics lead with a 54% market share in 2023, known for their rapid, sensitive, and specific disease detection capabilities.
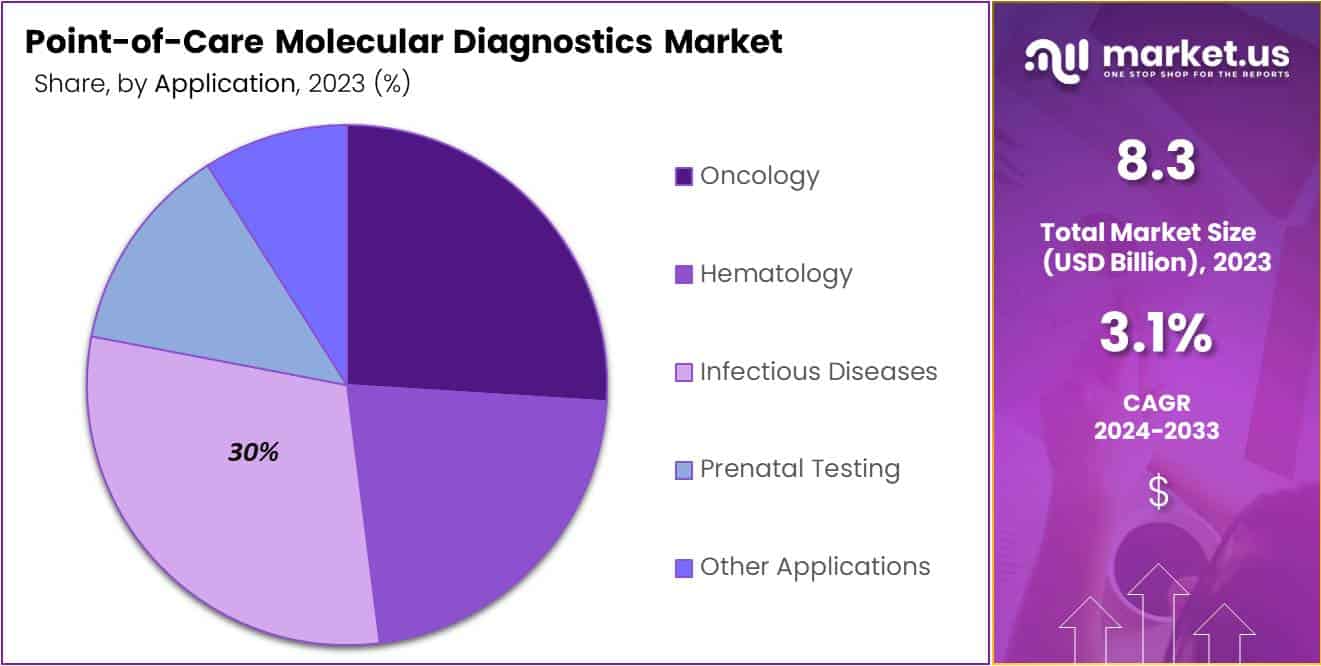
- Infectious diseases hold over 30% of the market, driven by the rising demand for rapid diagnostics.
- Over-The-Counter (OTC) diagnostics dominate the test location segment, capturing 51.7% of the market share due to the need for accessible healthcare.
- Decentralized Labs secure over 33% of the market share in the end-use segment, indicating a shift towards immediate diagnostic services.
- North America leads the global market with a 38% share in 2023, supported by advanced healthcare infrastructure and regulatory frameworks.
Get Sample PDF Report: https://market.us/report/point-of-care-molecular-diagnostics-market/request-sample/
Point-of-Care Molecular Diagnostics Market Key Segments
Technology
- PCR-based
- Hybridization-based
- Genetic Sequencing-based
- Microarray-based
Application
- Oncology
- Hematology
- Infectious Diseases
- Prenatal Testing
- Other Applications
Test Location
- OTC
- POC
End-use
- Hospitals
- Home-care
- Decentralized Labs
- Research Institutes
- Other End-Uses
Key Regions
- North America (The US, Canada, Mexico)
- Western Europe (Germany, France, The UK, Spain, Italy, Portugal, Ireland, Austria, Switzerland, Benelux, Nordic, Rest of Western Europe)
- Eastern Europe (Russia, Poland, The Czech Republic, Greece, Rest of Eastern Europe)
- APAC (China, Japan, South Korea, India, Australia & New Zealand, Indonesia, Malaysia, Philippines, Singapore, Thailand, Vietnam, Rest of APAC)
- Latin America (Brazil, Colombia, Chile, Argentina, Costa Rica, Rest of Latin America)
- Middle East & Africa (Algeria, Egypt, Israel, Kuwait, Nigeria, Saudi Arabia, South Africa, Turkey, United Arab Emirates, Rest of MEA)
Buy Directly: https://market.us/purchase-report/?report_id=117982
Key Players Analysis
F. Hoffmann-La Roche AG, a global leader in in-vitro diagnostics, is advancing point-of-care (POC) molecular diagnostics by integrating innovative technologies. The company recently acquired LumiraDx’s POC technology, which enhances their portfolio with versatile platforms for rapid and reliable testing. This acquisition aligns with Roche’s goal of decentralizing healthcare, making diagnostic tests more accessible and affordable. Roche’s POC molecular diagnostics cover a wide range of disease areas, including oncology and infectious diseases, leveraging PCR and other advanced technologies to deliver timely and accurate results across various healthcare settings.
Abbott Laboratories is a prominent player in the point-of-care molecular diagnostics market, known for its strong focus on developing rapid and accurate diagnostic solutions. Abbott’s molecular diagnostics portfolio includes tests for infectious diseases, oncology, and more, utilizing technologies like PCR and isothermal amplification. Their ID NOW platform, for instance, delivers COVID-19 test results in minutes, emphasizing speed and reliability. Abbott’s extensive global presence and continuous innovation make them a key contributor to the growing POC molecular diagnostics market.
QIAGEN N.V. is a leading provider of molecular diagnostics solutions, known for its comprehensive range of sample-to-insight technologies. In the POC molecular diagnostics sector, QIAGEN offers platforms such as the QIAstat-Dx, which provides rapid syndromic testing for infectious diseases. This system integrates multiple tests on a single platform, enabling healthcare professionals to make quick, informed decisions. QIAGEN’s focus on integrating advanced genetic sequencing and PCR technologies ensures high accuracy and efficiency in diagnostic processes, supporting the broader adoption of POC diagnostics globally.
Bayer AG is expanding its footprint in the point-of-care molecular diagnostics market, focusing on innovative solutions for rapid and accurate disease detection. Bayer’s efforts include leveraging CRISPR and next-generation sequencing (NGS) technologies to enhance diagnostic capabilities, particularly in oncology. Their commitment to research and development in molecular diagnostics aims to improve early detection and patient outcomes. Bayer’s integration of cutting-edge technologies positions them as a significant player in the POC molecular diagnostics landscape, addressing the growing demand for efficient and accessible diagnostic tools.
Nova Biomedical specializes in developing advanced point-of-care diagnostic solutions, focusing on critical and primary care settings. Their POC molecular diagnostics include platforms like the StatStrip and StatSensor, which provide rapid testing for glucose and other critical biomarkers. Nova Biomedical’s emphasis on accuracy, ease of use, and quick turnaround times ensures that their diagnostic tools meet the needs of healthcare professionals in various settings. Their continuous innovation and focus on improving patient care through reliable diagnostics make them a notable player in the POC molecular diagnostics market.
Point-of-Care Molecular Diagnostics Market Key Players:
- F. Hoffmann-La Roche AG
- Abbott Laboratories
- QIAGEN AV
- Bayer AG
- Nova Biomedical
- Danaher
- Nipro Diagnostics
- Bio-Rad Laboratories Inc.
- Agilent Technologies Inc.
- bioMérieux
- OraSure Technologies
Point-of-Care Molecular Diagnostics Market Report Scope >> Market Value (2023): USD 8.3 Billion || Forecast Revenue (2033): USD 11.3 Billion || CAGR (2024-2033): 3.1% || Base Year Estimation: 2023 || Historic Period: 2019-2022 || Forecast Period: 2024-2033.
Inquire More about report: https://market.us/report/point-of-care-molecular-diagnostics-market/#inquiry
About Market.US
Market.US is renowned for its comprehensive market research and analysis, providing customized and syndicated reports to a global clientele. Specializing in a variety of sectors, they offer strategic insights and detailed market forecasts, assisting businesses in making informed decisions. With a focus on innovation and accuracy, Market.US supports clients in over 126 countries, and maintains a strong repeat customer rate, underscoring their commitment to quality and client satisfaction. Their team excels in delivering exceptional research services, ensuring that no detail is overlooked in any target market.
Contact Details
Market.us (Powered By Prudour Pvt. Ltd.)
Contact No: +1 718 618 4351.
Email: [email protected]
Blog: https://medicalmarketreport.com/
View More Trending Reports
Endoscopic Closure Systems Market Will Reach USD 573.3 Million by 2033 and hit around 6.4% CAGR
Anticoagulant Market Set to Surge to USD 66.1 Billion by 2033
DNA Origami Market to Reach USD 105.2 Billion by 2033
Point-of-Care Molecular Diagnostics Market Estimated to Reach USD 11.3 Billion by 2033