The Global Menkes Syndrome Market is anticipated to grow significantly over the next decade, with its market size projected to increase from USD 0.2 million in 2023 to USD 0.5 million by 2033, reflecting a compound annual growth rate (CAGR) of 8.5% during the forecast period from 2024 to 2033. This growth is driven by several key factors, including advancements in genetic testing and early diagnosis, which are crucial for effective treatment. The increased awareness and research into Menkes Syndrome have also contributed to market expansion, alongside the development of novel copper-based therapies.
However, the market faces substantial challenges, such as the rarity of the disorder and the limited availability of effective treatments. Menkes Syndrome, a genetic disorder affecting copper levels in the body, presents significant clinical management difficulties due to its complexity and the need for early intervention. Recent developments in treatment approaches, including the use of copper histidinate and other copper supplements, have shown promise in improving patient outcomes when administered early.
Additionally, the prevalence of Menkes Syndrome, which primarily affects males, and ongoing clinical trials focused on new therapeutic agents are expected to influence market dynamics positively. North America currently dominates the market, driven by advanced healthcare infrastructure and robust research initiatives aimed at rare genetic disorders.
Overall, the Menkes Syndrome Market is poised for growth, supported by scientific advancements and increased focus on genetic research, despite the inherent challenges in managing such a rare condition.
Key Takeaways
- The Menkes Syndrome market is projected to reach USD 0.5 million by 2033, growing at a CAGR of 8.5% from 2024 to 2033.
- Penicillamine dominates the market for managing Menkes Syndrome symptoms, holding a 46% market share in 2023.
- Oral administration captures 53% of the market in 2023 due to its ease of use and ongoing innovations.
- Hospitals dominate the end-user segment, accounting for 43% of the market share in 2023 by providing comprehensive care.
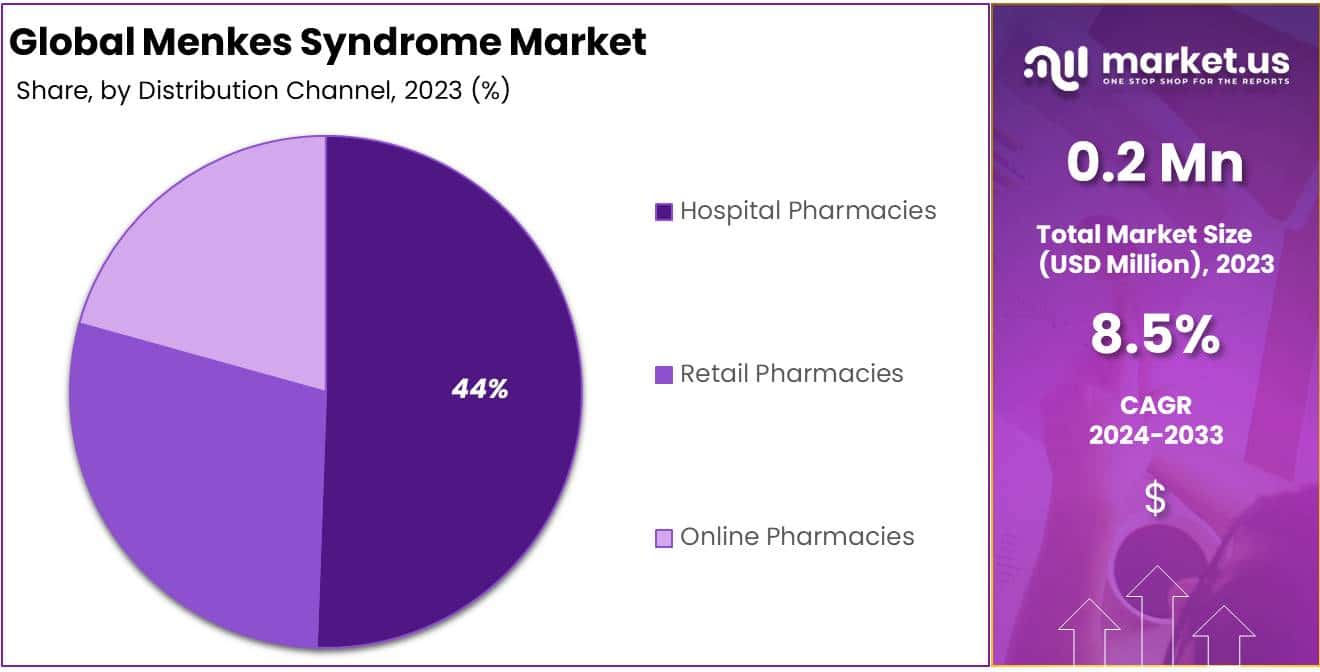
- Hospital pharmacies lead the distribution channels with a 44% share in 2023, ensuring access to specialized treatments.
- North America holds a 38% market share in 2023, valued at USD 0.07 million, driven by its advanced healthcare infrastructure.
Get Sample PDF Report: https://market.us/report/menkes-syndrome-market/request-sample/
Menkes Syndrome Market Key Segments
Drug Class
- Penicillamine
- Droxidopa
- Other Drug Classes
Route of Administration
- Oral
- Parenteral
End-User
- Hospitals
- Specialty Clinics
- Homecare
- Other End-Users
Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
Key Regions
- North America (The US, Canada, Mexico)
- Western Europe (Germany, France, The UK, Spain, Italy, Portugal, Ireland, Austria, Switzerland, Benelux, Nordic, Rest of Western Europe)
- Eastern Europe (Russia, Poland, The Czech Republic, Greece, Rest of Eastern Europe)
- APAC (China, Japan, South Korea, India, Australia & New Zealand, Indonesia, Malaysia, Philippines, Singapore, Thailand, Vietnam, Rest of APAC)
- Latin America (Brazil, Colombia, Chile, Argentina, Costa Rica, Rest of Latin America)
- Middle East & Africa (Algeria, Egypt, Israel, Kuwait, Nigeria, Saudi Arabia, South Africa, Turkey, United Arab Emirates, Rest of MEA)
Buy Directly: https://market.us/purchase-report/?report_id=77277
Key Players Analysis
Fortress Biotech is actively involved in developing treatments for Menkes Syndrome, a rare genetic disorder affecting copper transport. Their efforts are centered around AAV-ATP7A gene therapy, designed to deliver functional copies of the copper transporter gene. This therapy, combined with CUTX-101 (Copper Histidinate), has shown promising results in improving neurodevelopmental outcomes and survival rates in early-treated patients. Fortress Biotech collaborates with institutions like the NIH and Nationwide Children’s Hospital to advance these innovative therapies, which currently have no FDA-approved treatment options.
Teva Pharmaceutical Industries Ltd., a major player in the pharmaceutical industry, is exploring opportunities in the Menkes Syndrome market. While specific treatments for Menkes are not yet in their portfolio, Teva’s expertise in genetic and neurological disorders positions them to potentially develop therapies for this condition. The company’s extensive research and development capabilities and their strategic focus on innovative treatments could significantly impact the future management of Menkes Syndrome.
Amerigen Pharmaceuticals Limited, known for its generic pharmaceutical products, is positioned to provide affordable treatment options for Menkes Syndrome. Their focus on cost-effective solutions could improve access to essential medications for patients with this rare genetic disorder. Although specific details about their involvement in Menkes Syndrome treatments are limited, their potential role in this market aligns with their broader mission to enhance drug accessibility globally.
Mylan N.V., now part of Viatris, brings extensive experience in generic and specialty pharmaceuticals to the Menkes Syndrome market. Their capabilities in manufacturing and distributing complex generics and specialty drugs could support the development and availability of treatments for Menkes Syndrome. Mylan’s commitment to addressing unmet medical needs positions them well to contribute to this niche market, although specific products targeting Menkes Syndrome are still in development stages.
Bausch Health focuses on diverse therapeutic areas, including neurological disorders, which could extend to Menkes Syndrome treatments. Their expertise in developing and commercializing complex medications positions them as a potential key player in this market. While specific initiatives for Menkes Syndrome are not highlighted, Bausch Health’s strategic focus on innovation and addressing rare diseases aligns with the growing need for effective Menkes Syndrome therapies.
Menkes Syndrome Market Key Players:
- Fortress Biotech
- Teva Pharmaceutical Industries Ltd.
- Amerigen Pharmaceuticals Limited
- Mylan N.V.
- Bausch Health
- H. Lundbeck A/S
- Other Key Players
Menkes Syndrome Market Report Scope >> Market Value (2023): USD 0.2 Million || Forecast Revenue (2033): USD 0.5 Million || CAGR (2024-2033): 8.5% || Base Year Estimation: 2023 || Historic Period: 2019-2022 || Forecast Period: 2024-2033.
Inquire More about report: https://market.us/report/menkes-syndrome-market/#inquiry
About Market.US
Market.US is renowned for its comprehensive market research and analysis, providing customized and syndicated reports to a global clientele. Specializing in a variety of sectors, they offer strategic insights and detailed market forecasts, assisting businesses in making informed decisions. With a focus on innovation and accuracy, Market.US supports clients in over 126 countries, and maintains a strong repeat customer rate, underscoring their commitment to quality and client satisfaction. Their team excels in delivering exceptional research services, ensuring that no detail is overlooked in any target market.
Contact Details
Market.us (Powered By Prudour Pvt. Ltd.)
Contact No: +1 718 618 4351.
Email: [email protected]
Blog: https://medicalmarketreport.com/
View More Trending Reports
Cystoscope Market Size, Share and Growth Opportunities
AI In Animal Health Market Will Grow Nearly USD 7,477.9 million at a rate of 20.1% by 2033
AI In Pathology Market Predicted USD 119.0 Billion by 2033, An approximate 15.9% CAGR Growth
Augmented and Virtual Reality in Healthcare Market to Reach USD 19.15 Billion by 2033