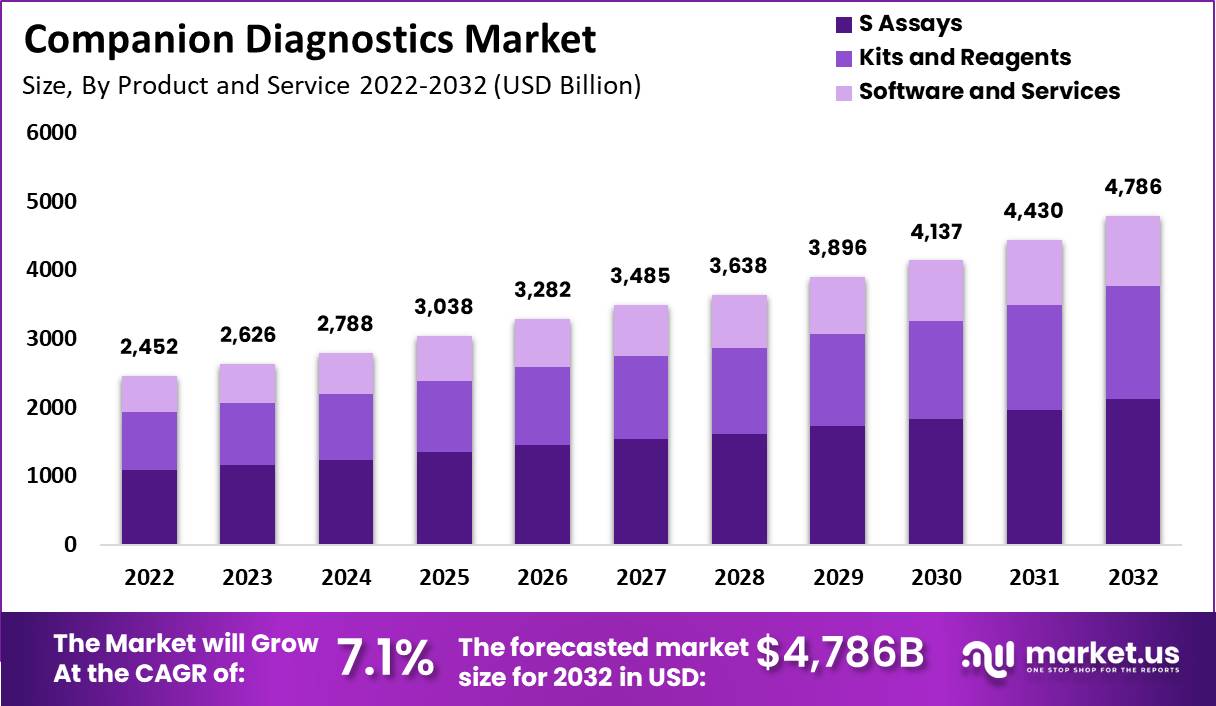
The Companion Diagnostics Market is projected to grow significantly, with its size expected to reach USD 4,786 million by 2032 from USD 2,452 million in 2022, at a CAGR of 7.1% during the forecast period. This growth is driven by several factors including the rising prevalence of chronic diseases, particularly cancer, which necessitates targeted therapies and personalized medicine. Technological advancements, such as the development of next-generation sequencing (NGS) and polymerase chain reaction (PCR) kits, are enhancing the accuracy and effectiveness of companion diagnostics.
Recent developments in the market highlight the FDA approvals for various CDx tests, such as Roche’s Cobas EGFR Mutation Test v2 for non-small cell lung cancer and Thermo Fisher Scientific’s Oncomine Dx Target Test for multiple tumor types. However, the market faces challenges like high capital investment, complex regulatory processes, and a challenging cost-benefit ratio due to the specialized nature of these tests.
Furthermore, significant investments by major companies such as Roche, Abbott Laboratories, and Thermo Fisher Scientific are expected to fuel market expansion. Government policies and collaborations between pharmaceutical companies and diagnostic manufacturers are also enhancing market growth.
Key Takeaways
- The global companion diagnostics market is anticipated to exceed USD 4,786 million by 2032, growing from USD 2,452 million in 2022.
- The companion diagnostics market is experiencing a robust growth rate, with a CAGR of 7.1%.
- In 2022, North America dominated the companion diagnostics market, holding a 43% revenue share.
- Europe accounted for a 25% revenue share in the companion diagnostics market in 2022.
- The Asia-Pacific region is expected to see significant growth due to rising cancer rates and better healthcare infrastructure.
- Market growth is driven by increased patient awareness, prevalence of chronic diseases, allergies, and personalized medicine advancements.
- The assay segment leads the market in revenue generation by product and services.
- Polymerase Chain Reaction (PCR) technology dominates the companion diagnostics market and is rapidly growing.
- Cancer care advancements and technological innovations are crucial factors in the indication segment.
- Pharmaceutical and biopharmaceutical companies hold the largest market share by end-users.
- Market dynamics are influenced by the demand for cost-effective, quality care and strict regulatory compliance.
- Increasing chronic disease cases boost the need for precise diagnosis and effective treatment.
- The emphasis on patient-centered care enhances the demand for companion diagnostics.
- Next-generation sequencing emerges as a significant trend in the global companion diagnostics market.
- Leading market players include Abbott, Roche, Illumina, and Thermo Fisher Scientific.
Get Sample PDF Report: https://market.us/report/companion-diagnostics-market/request-sample/
Companion Diagnostics Market Key Segments
By Product and Service
- S Assays
- Kits and Reagents
- Software and Services
By Technology
- Polymerase Chain Reaction
- Next Generation Sequencing
- In Situ Hybridization
- Immunohistochemistry
By Indication
- Lung Cancer
- Breast Cancer
- Colorectal Cancer
- Leukemia
- Melanoma
By End-User
- Pharmaceutical and Biopharmaceutical Companies
- Reference Laboratories
- Contract Research Organizations
Key Regions
- North America (The US, Canada, Mexico)
- Western Europe (Germany, France, The UK, Spain, Italy, Portugal, Ireland, Austria, Switzerland, Benelux, Nordic, Rest of Western Europe)
- Eastern Europe (Russia, Poland, The Czech Republic, Greece, Rest of Eastern Europe)
- APAC (China, Japan, South Korea, India, Australia & New Zealand, Indonesia, Malaysia, Philippines, Singapore, Thailand, Vietnam, Rest of APAC)
- Latin America (Brazil, Colombia, Chile, Argentina, Costa Rica, Rest of Latin America)
- Middle East & Africa (Algeria, Egypt, Israel, Kuwait, Nigeria, Saudi Arabia, South Africa, Turkey, United Arab Emirates, Rest of MEA)
Buy Directly: https://market.us/purchase-report/?report_id=57777
Key Players Analysis
Abbott is a key player in the companion diagnostics sector, leveraging its advanced molecular testing technologies to support personalized medicine. The company collaborates with biopharmaceutical firms like Celgene and Agios Pharmaceuticals to develop diagnostic tests that identify specific genetic mutations, such as IDH1 and IDH2, in leukemia patients. These tests are crucial for determining patient eligibility for targeted therapies, thereby enhancing treatment efficacy and patient outcomes. Abbott’s robust diagnostic platforms, including the m2000 RealTime System, are integral to these partnerships, showcasing the company’s commitment to advancing precision medicine.
Agilent Technologies is a prominent player in the companion diagnostics market, specializing in developing and supplying instruments and reagents for molecular diagnostics. The company focuses on providing solutions that enable personalized treatments, particularly in oncology. Agilent collaborates with pharmaceutical companies to create tests that identify biomarkers indicative of specific cancers, thereby guiding therapy decisions. Their comprehensive portfolio includes genomic analysis tools and automated laboratory systems, which enhance the accuracy and efficiency of diagnostic testing, solidifying their position in the market.
F. Hoffmann-La Roche is a leader in the companion diagnostics industry, known for its innovative diagnostic solutions that support personalized healthcare. The company offers a wide range of diagnostic tools, including the cobas platform, which is used for detecting genetic mutations and other biomarkers. Roche’s companion diagnostics are pivotal in identifying patients who are likely to benefit from specific therapies, particularly in oncology. Their strategic collaborations with pharmaceutical companies ensure the development of targeted treatments, reinforcing Roche’s commitment to precision medicine.
Guardant Health specializes in liquid biopsy technologies, providing non-invasive diagnostic tests that detect cancer-related genetic mutations from blood samples. Their flagship product, Guardant360, is widely used in clinical practice to guide treatment decisions for patients with advanced cancers. Guardant Health’s innovative approach allows for real-time monitoring of tumor dynamics, offering significant advantages over traditional tissue biopsies. The company’s focus on leveraging genomic data to inform treatment strategies underscores their role in advancing precision oncology and improving patient outcomes.
QIAGEN is a leading provider of sample and assay technologies for molecular diagnostics. In the companion diagnostics sector, QIAGEN develops and supplies tests that detect genetic mutations and other biomarkers critical for guiding targeted therapies. The company’s collaboration with pharmaceutical firms ensures the integration of their diagnostic tools into drug development pipelines. QIAGEN’s products, such as the therascreen assays, are designed to optimize treatment decisions in oncology, enhancing the efficacy of personalized medicine approaches and reinforcing their market presence.
Companion Diagnostics Market Key Players:
- Abbott (U.S.)
- Agilent Technologies Inc. (U.S.)
- F. Hoffmann-La Roche Ltd. (Switzerland)
- Guardant Health (U.S.)
- QIAGEN (Germany)
- Myriad Genetics, Inc. (U.S.)
- Illumina Inc. (U.S.)
- Thermo Fisher Scientific Inc. (U.S.)
- BIOMERIEUX (France)
- Myriad Genetics Inc. (U.S.)
- Other Key Players.
Companion Diagnostics Market Report Scope >> Market Value (2022): USD 2,452 Million || Forecast Revenue (2032): USD 4,786 Million || CAGR (2023-2032): 7.1% || Base Year Estimation: 2023 || Historic Period: 2019-2022 || Forecast Period: 2024-2033.
Inquire More about report: https://market.us/report/companion-diagnostics-market/#inquiry
About Market.US
Market.US is renowned for its comprehensive market research and analysis, providing customized and syndicated reports to a global clientele. Specializing in a variety of sectors, they offer strategic insights and detailed market forecasts, assisting businesses in making informed decisions. With a focus on innovation and accuracy, Market.US supports clients in over 126 countries, and maintains a strong repeat customer rate, underscoring their commitment to quality and client satisfaction. Their team excels in delivering exceptional research services, ensuring that no detail is overlooked in any target market.
Contact Details
Market.us (Powered By Prudour Pvt. Ltd.)
Contact No: +1 718 618 4351.
Email: [email protected]
Blog: https://medicalmarketreport.com/
View More Trending Reports
Urinary Drainage Bags Market Will Increase USD 4.9 Billion By 2032
Neuroendoscopy Devices Market Size Set To Reach USD 326 Million By 2032
Gout Therapeutics Market Increase USD 5.4 Billion By 2032
Dermatophytic Onychomycosis Treatment Market Size, Share and CAGR 8.46%
Bleeding Disorder Testing Market Will Reach USD 16.3 Billion By 2032