Clinical Trials Market Upcoming Trends & Business Opportunities
The Global Clinical Trials Market latest research report is published by Market.Us. In this report, you will find an analysis of the impact of the recent market disruptions such as the Russo-Ukrainian War and Covid-19 on the market. The report provides a qualitative analysis of the market using different frameworks like Porter and PESTLE analysis. The Clinical Trials Market report provides detailed segmentation and market size data by category, product type, application, and geography. The report also provides a comprehensive analysis of the key issues, trends and drivers, restraints and challenges, the competitive landscape, and recent developments like mergers and acquisitions in the market.
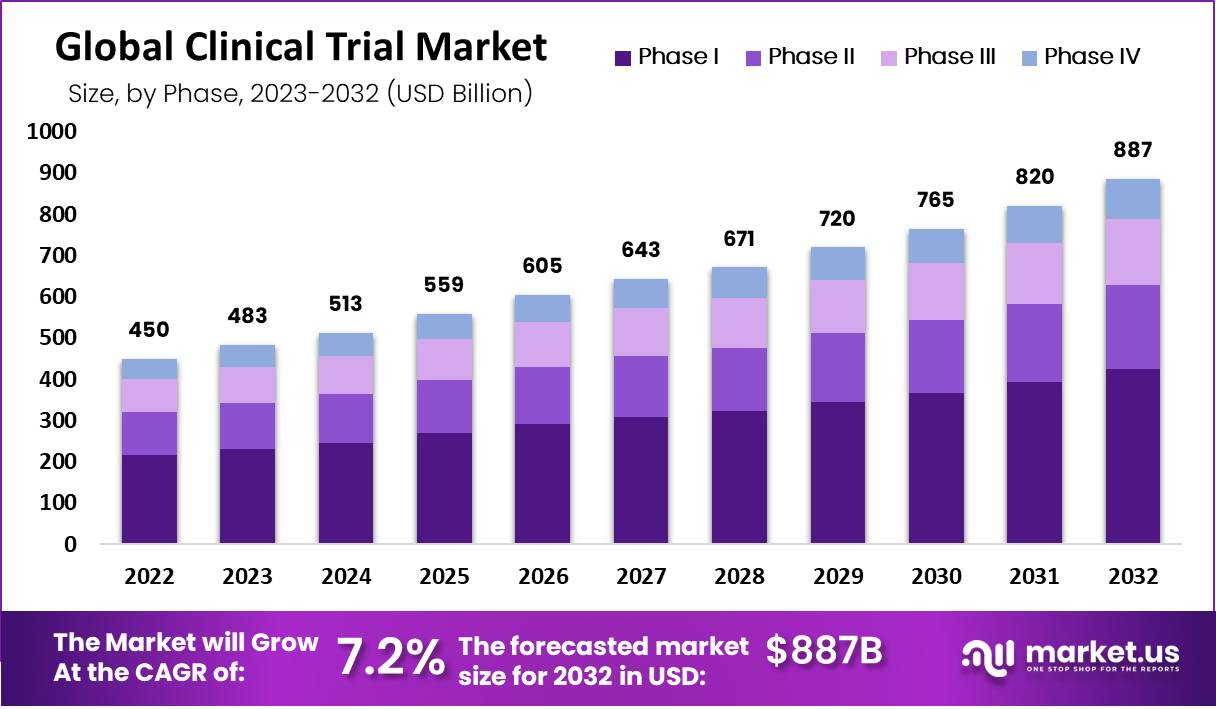
The Global Clinical Trials Market Was Valued at USD 450.1 Billion In 2022 and Is Expected To Reach USD 886.5 Billion by 2032, This Market Is Estimated To Register a CAGR Of 7.2%.
The Clinical Trials Market report provides a detailed analysis of current market trends to assess how these may impact the growth of the market. Additionally, the Clinical Trials Market encompasses an in-depth analysis of the global and regional markets along with a country-level market size breakdown to identify opportunities, challenges and better understand the market posture.
For insights on global, regional, and country-level parameters with growth opportunities from 2023 to 2032 – Download a sample report @ https://market.us/report/clinical-trials-market/request-sample/
The Clinical Trials marketplace is a dynamic and complex ecosystem where buyers and sellers interact to exchange goods, services, or goods. It serves as the backbone of economic activity, supporting trade, competition, and growth. In the Clinical Trials market, prices are determined by the forces of supply and demand and reflect the collective decisions of consumers and producers. Markets can range from small local exchanges to interconnected global networks spanning multiple industries and sectors.
The efficiency and effectiveness of the Clinical Trials market are influenced by factors such as competition, regulation, consumer preferences, technological advances, and economic conditions. In addition, markets facilitate the allocation of resources and offer companies the opportunity to innovate, grow and meet changing customer needs. Understanding Clinical Trials market dynamics is crucial for businesses, policymakers, and investors as it enables them to manage uncertainty, make informed decisions and respond to the ever-changing business landscape.
Top Clinical Trials Market Segments
Based on Phase
Phase I
Phase II
Phase III
Phase IV
Based on Indication
Pain Management
Oncology
CNS Condition
Diabetes
Obesity
Based on End-User
Pharmaceutical & Biopharmaceutical Companies
Clinical Research Organizations
Healthcare Providers
Top Clinical Trials Market Companies
Eli Lilly and Company
Parexel International Corporation
Pfizer
Charles River Laboratory
Syneous Health
Novo Nordisk A/S
IQVIA
ICON Plc.
Other Key Players.
Clinical Trials Market Regional Analysis
-North America [United States, Canada, Mexico]
-South America [Brazil, Argentina, Columbia, Chile, Peru]
-Europe [Germany, UK, France, Italy, Russia, Spain, Netherlands, Turkey, Switzerland]
-Middle East & Africa [GCC, North Africa, South Africa]
-Asia-Pacific [China, Southeast Asia, India, Japan, Korea, Western Asia]
You Can Directly Purchase This Report From Here: https://market.us/purchase-report/?report_id=66238
Market Dynamics
Drivers
- Increasing Prevalence of Chronic Diseases: The rising incidence of chronic diseases such as cancer, cardiovascular disorders, and diabetes drives the demand for clinical trials to develop effective treatments and therapies.
- Technological Advancements: Advancements in medical technology and data analytics have improved trial efficiency, patient recruitment, and data management, accelerating the overall clinical trial process.
- Growing Demand for Personalized Medicine: The shift towards personalized medicine necessitates more clinical trials to tailor treatments based on individual patient characteristics, driving the demand for targeted therapies.
- Regulatory Support and Streamlined Approval Processes: Supportive government regulations and initiatives aimed at speeding up approval processes have encouraged pharmaceutical companies to conduct more clinical trials, boosting market growth.
Restraints
- High Costs and Lengthy Trial Timelines: Clinical trials are expensive and time-consuming endeavors, involving substantial financial investments and extended periods for conducting trials, leading to a restraint on market growth.
- Stringent Regulatory Requirements: Strict regulatory compliance and ethical considerations can pose challenges for companies, often leading to delays or even trial cancellations.
- Recruitment and Retention of Participants: Finding and retaining suitable participants for clinical trials can be challenging, especially for rare diseases, potentially causing delays and impacting trial outcomes.
- Safety Concerns: The safety of participants is of utmost importance, and any adverse events or complications during a trial can lead to legal and reputational risks for the sponsoring companies.
Opportunities
- Rise of Virtual and Decentralized Trials: Advancements in telemedicine and digital health technologies offer opportunities for conducting virtual or decentralized clinical trials, enhancing patient participation and reducing costs.
- Emergence of Real-World Evidence (RWE): The increasing utilization of real-world data in clinical research provides an opportunity to complement traditional clinical trial data, leading to more comprehensive insights.
- Collaborations and Partnerships: Strategic collaborations between pharmaceutical companies, research organizations, and academic institutions can foster innovation and expedite clinical trial processes.
- Focus on Rare Diseases and Orphan Drugs: Governments’ incentives and market exclusivity for orphan drugs encourage more clinical trials focused on rare diseases, representing an untapped opportunity.
Challenges
- COVID-19 Pandemic Impact: The pandemic disrupted ongoing trials, delayed new trials, and diverted resources, posing significant challenges to the conduct of clinical trials worldwide.
- Data Management and Privacy Concerns: The large volume of data generated during clinical trials requires robust management systems while ensuring patient privacy and data security.
- Complexity of Trial Designs: The increasing complexity of clinical trial protocols can result in operational challenges, resource constraints, and potential difficulties in data interpretation.
- Competition and Patent Expirations: Intense competition in the pharmaceutical industry and patent expirations of blockbuster drugs can affect the willingness of companies to invest in new trials.
What is included in the Clinical Trials Market Report Access?
• This report provides quantitative analysis of Clinical Trials market segments, recent trends, estimates, and market analysis dynamics from 2023 to 2032 to identify the market leaders & market opportunities.
• Market studies are offered with information on the main drivers, constraints, and opportunities.
• Porter’s five forces analysis underscores the potential of buyers and suppliers to empower stakeholders to make profit-oriented business decisions and to strengthen their supplier-buyer networks.
• In-depth analysis of market segmentation Clinical Trials helps to identify dominant market opportunities.
• The top countries of each region are shown based on their global market share of sales.
• The positioning of market participants facilitates benchmarking and provides a clear understanding of the current position of market participants.
• The report provides an analysis of regional and global Clinical Trials market trends, key players, market segments, application areas and market development strategies.
Key Topics Covered
1. summary
2. Clinical Trials Market Characteristics
3. Clinical Trials Market Trends and Strategies
4. Impact of COVID-19 on Clinical Trials
5. Clinical Trials Market Size and Growth
6. Clinical Trials Market segmentation
7. Clinical Trials Regional and National Market Analysis
8. Clinical Trials Market Competition and Company Profiles
9. Major Mergers and Acquisitions in the Clinical Trials Market
10. Clinical Trials Future Prospects of Market and Potential Analysis
11. Appendix
For insights on global, regional, and country-level parameters with growth opportunities from 2023 to 2032 – Download a sample report @ https://market.us/report/clinical-trials-market/request-sample/
Get in Touch with Us:
Business Development Team – Market.us
Market.us (Powered By Prudour Pvt. Ltd.)
Address: 420 Lexington Avenue, Suite 300 New York City, NY 10170, United States
Tel: +1 718 618 4351
Send Email: [email protected]