The global pharmacovigilance market is projected to expand significantly, with its size expected to reach around USD 19 billion by 2032 from USD 7.8 billion in 2022, growing at a CAGR of 9.3% during the forecast period from 2022 to 2032. This growth is driven by several key factors, including the increasing incidence of adverse drug reactions (ADRs) and the rising consumption of pharmaceuticals due to the prevalence of chronic diseases such as hypertension, diabetes, and cardiac disorders. The demand for robust drug safety and monitoring systems has intensified, prompting advancements in automated ADR reporting platforms and comprehensive pharmacovigilance processes.
Recent developments highlight the shift towards outsourcing pharmacovigilance operations to third-party service providers, enhancing flexibility and efficiency in drug safety monitoring. The phase IV (post-marketing) segment, crucial for detecting long-term adverse effects, remains dominant, contributing significantly to market revenue. Additionally, the oncology segment is rapidly growing due to increased research and government initiatives aimed at improving cancer treatment outcomes.
Challenges in the pharmacovigilance market include managing the complex regulatory requirements and ensuring the accuracy and consistency of adverse event reporting across different regions. Nonetheless, the integration of advanced technologies such as AI and machine learning in case data management is poised to streamline these processes and support the market’s growth.
Key Takeaways
- The global pharmacovigilance market is projected to reach USD 19 billion by 2032, with a CAGR of 9.3% during the forecast period.
- Increasing chronic diseases are boosting drug consumption, consequently raising the demand for pharmacovigilance services.
- Spontaneous reporting leads the market, benefiting from data simulation and effective drug comparison.
- Cohort event monitoring is gaining traction due to its capability in data mining and surveillance for both new and existing medicines.
- Targeted spontaneous reporting is the fastest-growing segment, driven by government initiatives promoting diverse reporting methods.
- The use of electronic health records (EHR) in risk identification post-hospital discharge is expanding this segment.
- Phase IV post-marketing surveillance is critical for detecting unexpected adverse drug reactions, dominating this market phase.
- Contract outsourcing is the fastest-growing service provider segment due to its cost-efficiency, flexibility, and resource-sharing benefits.
- The oncology market’s focus on cancer drug safety monitoring is a significant industry growth driver.
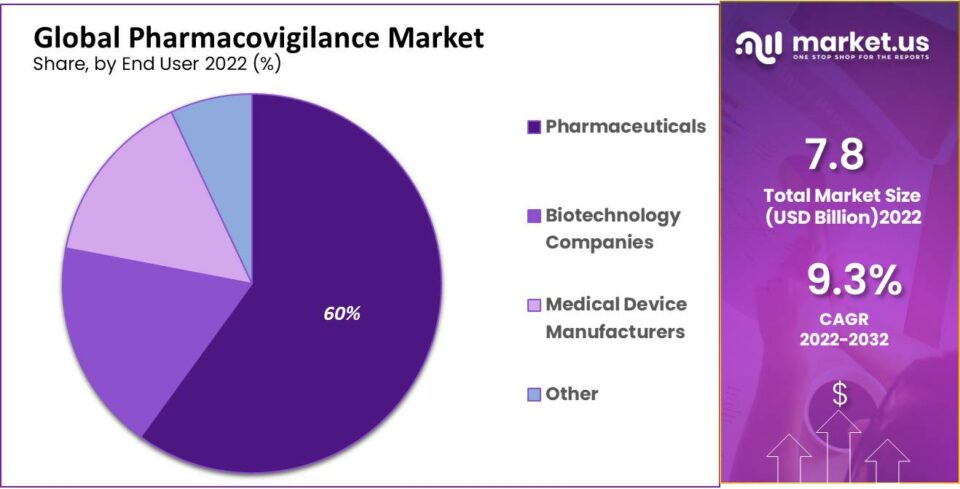
- Pharmaceutical companies hold a substantial market share, driven by the increased utilization of pharmacovigilance services.
Get Sample PDF Report: https://market.us/report/pharmacovigilance-market/request-sample/
Pharmacovigilance Market Key Segments
By Service Provider
- In-house
- Contract Outsourcing
- Others
By Product Life Cycle
- Pre-clinical
- Phase I
- Phase II
- Phase III
- Phase IV
By Type
- Spontaneous Monitoring
- Intensified ADR Monitoring
- Targeted Spontaneous Reporting
- Cohort Event Monitoring
- Others
By Process Flow
- Case Data Management
- Signal Detection
- Risk Management System
- Others
By Therapeutic Area
- Oncology
- Neurology
- Cardiology
- Pulmonology
- Others
Based By End-User
- Pharmaceuticals
- Biotechnology Companies
- Medical Device Manufacturers
- Others
Key Regions
- North America (The US, Canada, Mexico)
- Western Europe (Germany, France, The UK, Spain, Italy, Portugal, Ireland, Austria, Switzerland, Benelux, Nordic, Rest of Western Europe)
- Eastern Europe (Russia, Poland, The Czech Republic, Greece, Rest of Eastern Europe)
- APAC (China, Japan, South Korea, India, Australia & New Zealand, Indonesia, Malaysia, Philippines, Singapore, Thailand, Vietnam, Rest of APAC)
- Latin America (Brazil, Colombia, Chile, Argentina, Costa Rica, Rest of Latin America)
- Middle East & Africa (Algeria, Egypt, Israel, Kuwait, Nigeria, Saudi Arabia, South Africa, Turkey, United Arab Emirates, Rest of MEA)
Buy Directly: https://market.us/purchase-report/?report_id=96646
Key Players Analysis
Accenture Plc offers comprehensive pharmacovigilance services that span the entire drug safety lifecycle. Their operations are supported by over 1,200 dedicated pharmacovigilance professionals, including 60 physicians and 45 medical writers. The company processes around 1.5 million cases annually using advanced AI technologies integrated into their INTIENT Pharmacovigilance platform, which optimizes case processing and data management. This platform helps clients streamline pharmacovigilance processes, enhance patient safety, and comply with regulatory requirements. Accenture’s global delivery model ensures seamless, efficient operations and supports clients’ pharmacovigilance needs worldwide.
Bristol-Myers Squibb (BMS) collaborates with Accenture to operate a joint center for pharmacovigilance in Chennai, India. This center processes and codes adverse event data, generates regulatory reports, and conducts medical reviews. The collaboration leverages Accenture’s technological expertise to enhance BMS’s pharmacovigilance capabilities, ensuring compliance and improving patient safety outcomes. This partnership allows BMS to scale its operations efficiently while maintaining high standards in monitoring drug safety across its global operations.
Clinquest Group B.V. is known for its specialized pharmacovigilance services, providing robust solutions in drug safety monitoring. They offer end-to-end pharmacovigilance support, including adverse event reporting, risk management, and compliance services. Their team of experts ensures that clients meet regulatory requirements and maintain high standards of patient safety. Clinquest utilizes advanced technology and analytical tools to deliver precise and timely pharmacovigilance services, supporting pharmaceutical companies in their drug development and post-market surveillance activities.
Cognizant Technology Solutions Corporation delivers extensive pharmacovigilance services through its life sciences division. Their offerings include case processing, signal detection, risk management, and regulatory submissions. Cognizant leverages its technological expertise to automate and streamline pharmacovigilance processes, ensuring efficient handling of safety data. Their global network of professionals provides continuous support to pharmaceutical companies, helping them comply with international safety standards and enhance patient safety outcomes.
GlaxoSmithKline (GSK) focuses on comprehensive pharmacovigilance to ensure the safety of their pharmaceutical products. GSK employs advanced analytics and robust reporting systems to monitor adverse events and manage risks associated with drug use. Their pharmacovigilance strategies are integrated into the entire drug development lifecycle, from clinical trials to post-market surveillance. GSK’s commitment to patient safety is reflected in their proactive approach to pharmacovigilance, which includes continuous monitoring and improvement of safety protocols to meet global regulatory standards.
Pharmacovigilance Market Key Players:
- Accenture Plc
- Bristol-Myers Squibb Company
- Clinquest Group B.V.
- Cognizant Technology Solutions Corporation
- GlaxoSmithKline plc
- ICON plc
- Novartis AG
- Hoffmann-La Roche Ltd.
- PAREXEL International Corporation
- Pfizer Inc.
- ICON Plc
- Wipro Limited
Pharmacovigilance Market Report Scope >> Market Value (2022): USD 7.8 Billion || Forecast Revenue (2032): USD 19 Billion || CAGR (2023-2032): 9.3% || Base Year Estimation: 2023 || Historic Period: 2019-2022 || Forecast Period: 2024-2033.
Inquire More about report: https://market.us/report/pharmacovigilance-market/#inquiry
About Market.US
Market.US is renowned for its comprehensive market research and analysis, providing customized and syndicated reports to a global clientele. Specializing in a variety of sectors, they offer strategic insights and detailed market forecasts, assisting businesses in making informed decisions. With a focus on innovation and accuracy, Market.US supports clients in over 126 countries, and maintains a strong repeat customer rate, underscoring their commitment to quality and client satisfaction. Their team excels in delivering exceptional research services, ensuring that no detail is overlooked in any target market.
Contact Details
Market.us (Powered By Prudour Pvt. Ltd.)
Contact No: +1 718 618 4351.
Email: [email protected]
Blog: https://medicalmarketreport.com/
View More Trending Reports
Surgical Equipment Market Forecasted To Attain USD 36 Billion By 2033, Showcasing A 8.2% CAGR
Intraocular Lens Market Economic Projections Soar To USD 8,186 Million By 2033, With 6.3% CAGR
Needles Market Trends Suggest A USD 15.3 Billion Valuation By 2033, With A Projected 7.3% CAGR